
|

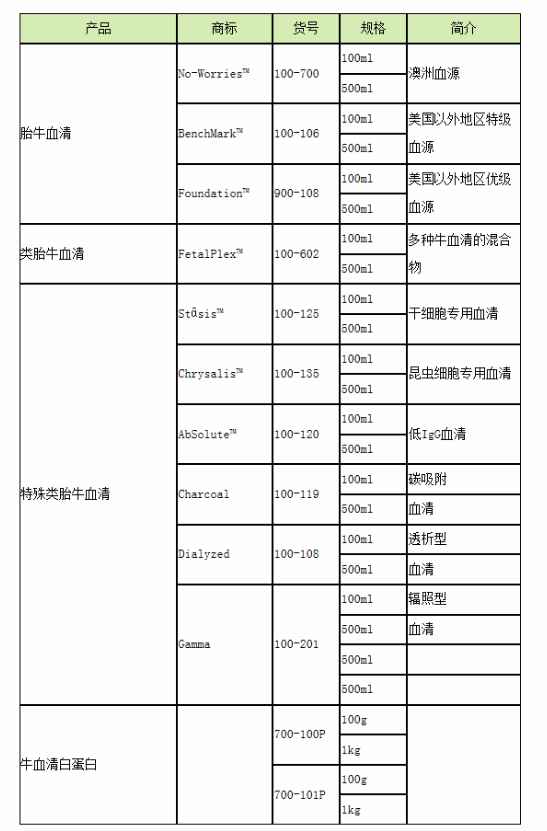
| 产地 | USA |
| 保存条件 | -20°C |
| 品牌 | GEMINI |
| 货号 | 900-108 |
| 用途 | 细胞培养 |
| 组织来源 | 胎牛 |
| 细胞形态 | 详见说明书 |
| 是否是肿瘤细胞 | 否 |
| 保质期 | 详见说明书 |
| 器官来源 | 胎牛 |
| 免疫类型 | 血清 |
| 品系 | 胎牛血清 |
| 生长状态 | 详见说明书 |
| 物种来源 | 详见说明书 |
| 包装规格 | 500ml |
| 是否进口 | 是 |
Gemini公司,成立于1985年,位于加利福尼亚南部Calabasas市,1999年,迁至加利福尼亚北部Woodland市。
Gemini始终以“为客户提供高质量产品”为首要目标尤其在细胞培养用胎牛血清方面,Gemini产品质量好、价格低和同类产品相比竞争
力强。
Gemini公司在销售的动物血清产品.全部来自干澳洲的子公司,血源主要为澳大利亚、新西兰、巴拿马、乌拉圭等。
PRODUCT DATASHEET
PRODUCT DESCRIPTION:
Gemini's FoundationM fetal bovine serum is intended for the customer who wants excellent cell growth while buving at the most economical prices.
t his material originates from USDA-approved sources and goes through the same filtration schemes and final quality-control testing as our other gra
des. This material is distinguished from our BenchMarkM FBS by allowing higher levels of hemoglobin and or endotoxin
?Mvcoplasma-tested and virus-screened
?0.1 um sterile-filtered
INSTRUCTIONS FORUSE
Receipt and Storage:
Ships on dryice. Store frozen at-20 to -10°C
Thawing Instructions:
When preparing animal serum for use. it should be allowed to thaw in a refrigerator at a temperature of 2-8℃ overnight to minimize thermodynamic
stress to the material
Appearance:
Straw-like or amber color. It could be slightly cloudy and may contain precipitates due to freezing and or thawing, this occurrence does not impact c
ulture performance.
Stability:
If stored frozen(-20 to -10°C) product expiration is listed on CoA
Preparation and Use:
We recommend that you minimize the number of freeze/thaw cycles the product will undergo by aliquoting the serum into useful volumes at time of
storage.
Our fetal bovine serum meets all USDA requirements and has passed ante and post-mortem inspection by a licensed veterinarian. It is collected in co
untries recognized as being free offoot-and-mouth disease and
rinderpest. The origin of all fetal bovine serum is traceable by lot number.date. and country of collection
Precipitates may develop in this product upon freezing and or thawing; this occurrence does not impact culture performance
*Testing not required if Bovine Viral Diarrhea(Cytopathic)and Bovine Viral Diarrheanon-cytopathic) is not detected
**Some geographic regions may test higher than 0.300mg/mL
Ifthis be the case, refer to GGT results less than 10 U/L to ensure FBS purity
For Cell Culture,further Manufacturing and Research use only.Not for direct Therapeutic use.